ST. PAUL, Minn.–(BUSINESS WIRE)–Cardiovascular Systems, Inc. (CSI®) (NASDAQ: CSII), a medical device company developing and commercializing innovative interventional treatment systems for patients with peripheral and coronary artery disease, announced today that results from a large retrospective observational study of coronary orbital atherectomy will be released at TCT Connect 2020. Severe calcification […]
Peripheral/Endo
PQ Bypass Completes Enrollment in DETOUR2 Percutaneous Femoral-Popliteal Bypass Pivotal Study for Patients With Complex Peripheral Arterial Disease
FDA-designated Breakthrough Device now one step closer to PMA submission MILPITAS, Calif.–(BUSINESS WIRE)–PQ Bypass, an innovative medical device company pioneering advancements in the treatment of complex peripheral artery disease (PAD), announces enrollment of the final subject in the company’s flagship IDE, the DETOUR2 clinical trial. This important milestone occurs only […]
Teleflex Announces Expanded Indications for the Arrow® EZ-IO® Intraosseous Vascular Access System
Now Cleared for up to 48-Hour Dwell WAYNE, Pa., Oct. 01, 2020 (GLOBE NEWSWIRE) — Teleflex Incorporated (NYSE: TFX), a leading global provider of medical devices for critical care and surgery, has announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to expand the Indications […]
Mentice to Acquire Vascular Simulations
– Combined portfolio will enhance Mentice’s endovascular simulation offerings with world class replication solutions – Acquisition to expand Mentice’s global customer base and offer a strong entry into previously untapped market segments STOCKHOLM, Oct. 1, 2020 /PRNewswire/ — Mentice AB (STO: MNTC) today signed a definitive agreement to acquire the New York-based medical […]
Vascular Surgeon Eugene Tanquilut Performs First-Ever “Ellipsys” Dialysis Access Procedure in Chicago Southland
Groundbreaking, minimally invasive procedure means dialysis in half the time for late stage renal failure patient TINLEY PARK, Ill.–(BUSINESS WIRE)–Vascular Specialists in Tinley Park, Illinois, is pleased to announce that Dr. Eugene Tanquilut, has successfully completed the first Ellipsys Vascular Access System procedure in the Chicago Southland region. “Peter Song was a perfect candidate […]
Preclinical Studies Reveal Balloon-Based Drug Delivery to Be Critically Dependent on Coating Micro-Morphology, Suggesting a Paradigm for Accelerated Treatment Optimization
“These preclinical studies transform our understanding of how drug coated balloons deliver drug to the artery and why there is no class effect, while also providing tools for understanding the role of tissue stiffening with disease. The combination of mechanistic insight with predictive modeling can help accelerate development of new […]
JanOne Successfully Begins Production of JAN101 cGMP Batch for Phase 2b Peripheral Artery Disease (PAD) Trial and Potential Covid-19 Study
Company continues to make progress on its potential treatment for PAD, a disease affecting 200 million people worldwide LAS VEGAS, Sept. 24, 2020 /PRNewswire/ — JanOne Inc. (Nasdaq: JAN), a company focused on developing treatments for conditions that cause severe pain and drugs with non-addictive, pain-relieving properties, today announced that the Company has started […]
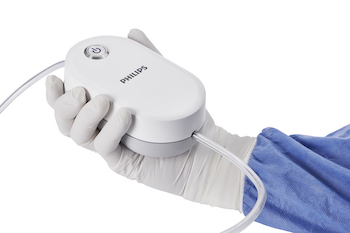
Philips expands peripheral vascular disease portfolio with the QuickClear mechanical thrombectomy system for blood clot removal
Powerfully simple thrombectomy solution provides physicians with an all-in-one, single-use aspiration catheter and pump system Intuitive design eliminates the need for capital equipment, with easy setup supporting faster procedures times Significantly smaller footprint provides the same or greater aspiration power as currently-available thrombectomy devices [1] Amsterdam, the Netherlands – Royal Philips (NYSE: […]
Gunze Provides Biodegradable Scaffolds for TEVG Clinical Trial to Be Conducted at the Abigail Wexner Research Institute at Nationwide Children’s Hospital to Improve Pediatric Patient Clinical Outcome
OSAKA, Japan–(BUSINESS WIRE)–Gunze Limited (Headquarters: Osaka, Japan, President: Atsushi Hirochi) [TOKYO:3002] is pleased to announce that Gunze’s biodegradable scaffolds are being provided to the Abigail Wexner Research Institute at Nationwide Children’s Hospital (Location: Columbus, OH, USA) to conduct its first human tissue engineered vascular grafts (hereinafter TEVG), clinical trial. 1. […]
Surmodics Receives FDA 510(k) Clearance for Pounce™ Thrombus Retrieval System
Next-generation technology provides easy, effective clot removal from peripheral arterial vasculature EDEN PRAIRIE, Minn.–(BUSINESS WIRE)–Surmodics, Inc. (NASDAQ:SRDX), a leading provider of medical device and in vitro diagnostic technologies to the health care industry, announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Pounce™ Thrombus Retrieval System. […]