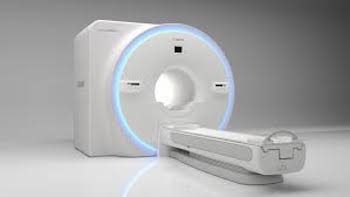
New Software Version for Vantage Orian 1.5T Also Offers Solution to Help Clinicians Image and Quantify Fatty Liver Disease TUSTIN, Calif.–(BUSINESS WIRE)–Hospitals and institutions are continually looking for ways to improve diagnostic imaging throughput, especially in today’s environment where disinfection of systems and rooms in between patients is crucial. Now, […]
Regulatory
FDA Approves Labeling Update for Abbott’s HeartMate 3 Heart Pump for use in Pediatric Patients
– Abbott’s HeartMate 3™ heart pump approved for use for pediatric patients battling advanced heart failure – This life-saving technology provides new treatment option for underserved population ABBOTT PARK, Ill., Dec. 17, 2020 /PRNewswire/ — Abbott (NYSE: ABT) today announced the U.S. Food and Drug Administration (FDA) has approved updated labeling for the […]
Occlutech’s Atrial Flow Regulator (AFR) Receives U.S. FDA Breakthrough Device Designation
SCHAFFHAUSEN, Switzerland, Dec. 18, 2020 /PRNewswire/ — Occlutech, a privately-held company, announced today that the US Food and Drug Administration (FDA) has granted the company a Breakthrough Device designation for its first-in-class, implantable Atrial Flow Regulator (AFR) for Pulmonary Arterial Hypertension (PAH). PAH affects hundreds of thousands in the U.S. and globally and […]
Small vessels can cause big problems; MagicTouch SCB Granted ‘Breakthrough Device Designation’ for the treatment of Small Coronary Artery Lesions
TAMPA, Fla., Dec. 17, 2020 /PRNewswire/ — Concept Medical Inc. (CMI) has been granted “Breakthrough Device Designation” from the Center for Devices and Radiological Health (CDRH) of the Food and Drug Administration (FDA) for the MagicTouch SCB Sirolimus Coated Balloon Catheter, for the treatment of small coronary artery lesions in Coronary Artery Disease (CAD). The proposed […]
FARAPULSE’s Pivotal Trial to Assess Its Leading Pulsed Field Ablation System for The Treatment of Atrial Fibrillation Receives FDA Conditional Approval
– Large, Randomized Controlled ADVENT Trial Designed to Establish New Gold Standard for AF Ablation – MENLO PARK, Calif., Dec. 17, 2020 /PRNewswire/ – FARAPULSE Inc. (“FARAPULSE” or “the Company”) today announced the U.S. Food and Drug Administration (FDA) conditionally approved the Company’s Investigational Device Exemption (IDE) application to initiate its U.S. pivotal ADVENT […]
Novartis announces positive FDA Advisory Committee recommendation for use of Entresto® to treat patients with HFpEF
The Committee voted 12 to 1 that the data presented support the use of Entresto in treatment of patients with heart failure with preserved ejection fraction (HFpEF) Potential Q1 2021 sNDA approval could make Entresto the first therapy indicated for use in treatment of patients with both major types of […]
FDA Clears Fever-Detecting Vital Data Sensor in New Cardiac Monitor
LAKE OSWEGO, Ore., Dec. 16, 2020 /PRNewswire/ — BIOTRONIK today announced FDA clearance and availability of the Vital Data Sensor, which identifies body temperature increases potentially associated with fever, in the new BIOMONITOR IIIm injectable cardiac monitor (ICM), as shown in a published case report1. Physicians will now have access to daily reports on […]
FDA Awards Genetesis Breakthrough Device Designation for CardioFlux® Imaging Technology
MASON, Ohio–(BUSINESS WIRE)–Genetesis, Inc. announced today that its flagship product, CardioFlux®, has been granted Breakthrough Device Designation by the U.S. Food and Drug Administration (FDA) for the diagnosis of myocardial ischemia and infarction in patients with symptoms suggestive of acute coronary syndrome. CardioFlux®, the most advanced commercially available magnetocardiograph (MCG), […]
Foldax Receives FDA Approval to Initiate Clinical Study of Biopolymer Mitral Heart Valve
SALT LAKE CITY–(BUSINESS WIRE)–Foldax®, Inc. today announced that the U.S. Food and Drug Administration (FDA) has granted IDE approval for the company to initiate a U.S. clinical study of its Tria™ biopolymer mitral surgical heart valve. The company anticipates the first use of its mitral valve in a human will […]
SoniVie Receives FDA Breakthrough Device Designation for the TIVUS System for Renal Artery Denervation
– SoniVie Acquires New Intellectual Property and Other Assets from Cardiosonic Moving TIVUS into New Therapeutic Areas Beyond Pulmonary Hypertension – TEL AVIV, Israel, Dec. 09, 2020 (GLOBE NEWSWIRE) — SoniVie, an Israeli company developing a novel proprietary Therapeutic Intra-Vascular Ultrasound (TIVUS) System to treat a variety of hypertensive disorders, […]