SILVER SPRING, Md., March 21, 2019 /PRNewswire/ — The U.S. Food and Drug Administration today approved the Optimizer Smart system for treating patients with chronic, moderate-to-severe heart failure who are not suited for treatment with other heart failure devices such as cardiac resynchronization therapy to restore a normal timing pattern of the heartbeat. […]
Rhythm
Correvio Announces Presentation Of Brinavess® Data At The American College Of Cardiology 2019 Annual Meeting
VANCOUVER, March 20, 2019 /PRNewswire/ – Correvio Pharma Corp. (NASDAQ: CORV) (TSX: CORV), a specialty pharmaceutical company focused on commercializing hospital drugs, today announced the presentation of new Brinavess® (vernakalant hydrochloride, IV) data at the American College of Cardiology 2019 Annual Meeting taking place March 16-18, 2019, in New Orleans. This poster presentation highlights reduced […]
Data from the Non-interventional EMIT-AF/VTE Study Shows Low Thromboembolic and Bleeding Event Rates in Unselected Elderly AF/VTE Patients on Oral, Once-daily LIXIANA®▼ Undergoing Diagnostic or Therapeutic Procedures
MUNICH, March 19, 2019 /PRNewswire/ — In 2019, the Edoxaban Clinical Research Programme will deliver new evidence on LIXIANA®▼ (edoxaban) use in clinical practice. EMIT-AF/VTE is one of the first sets of data to be presented EMIT-AF/VTE is a large observational, multicentre, multinational study on edoxaban peri-procedural management and outcomes It is […]
New Data for Abbott’s HeartMate 3™ Highlights Unparalleled Performance of Industry’s Leading Heart Pump
NEW ORLEANS, March 17, 2019 /PRNewswire/ — Abbott (NYSE: ABT) today announced new late-breaking data from the MOMENTUM 3 study, the world’s largest randomized controlled trial to assess outcomes in patients receiving a heart pump to treat advanced heart failure. In reviewing data across the complete 1,028 patient cohort, MOMENTUM 3 met its primary endpoint […]
Impella RP Post-Approval Study Data Presented at ACC 2019
March 18, 2019 07:00 AM Eastern Daylight Time NEW ORLEANS, La.–(BUSINESS WIRE)–Abiomed (NASDAQ:ABMD), a leading provider of breakthrough heart support technologies and the maker of the Impella RP heart pump, announces that survival data from the 18 month post-approval study of 42 Impella RP patients was presented at the American College of Cardiology’s […]
FDA Approves Industry’s Smallest, Slimmest 3T Tachycardia Devices from BIOTRONIK
LAKE OSWEGO, Ore., March 14, 2019 /PRNewswire/ — BIOTRONIK today announced FDA approval of the Acticor and Rivacor high-voltage cardiac rhythm management (CRM) device families for treatment of patients with cardiac arrhythmias. The six new tachycardia solutions include Rivacor VR-T, Rivacor DR-T, Rivacor HF-T QP, Acticor DX, Acticor CRT-DX Bipolar and Acticor […]
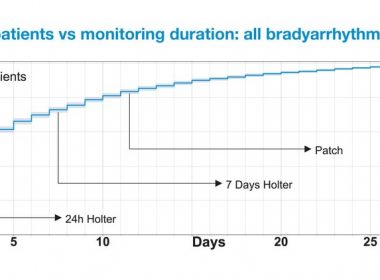
Study Finds Advantages of Full-disclosure Mobile Telemetry Monitoring over Patch and Holter Methods to Diagnose Patients with Bradyarrhythmia
NEW ORLEANS, March 14, 2019 /PRNewswire/ — Medi-Lynx Cardiac Monitoring, LLC, a leading provider of cardiac monitoring solutions, today announced results from a large retrospective analysis which demonstrated that cardiac monitoring with full-disclosure mobile cardiac telemetry (PocketECG) for up to 30 days significantly increased diagnostic yield for patients with bradyarrhythmia over Patch and […]
LindaCare to Debut ProPulse™ Cardiac Remote Monitoring Services at ACC in New Orleans
LEUVEN, Belgium and HARTFORD, Conn., March 14, 2019 /PRNewswire/ — LindaCare, a digital health company specializing in remote patient monitoring solutions for chronic disease management, today announced the company will offer ProPulse™, a new remote monitoring service for patients with cardiac implantable devices, adding to its online device management software solution. Dr. Robert Lerman, LindaCare’s Chief […]
Boston Scientific Receives CE Mark for Next Generation WATCHMAN FLX™ Left Atrial Appendage Closure Device
MARLBOROUGH, Mass., March 13, 2019 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) announced it has received CE Mark and initiated a limited market release of the next generation WATCHMAN FLX™ Left Atrial Appendage Closure (LAAC) Device in Europe. Patients with AF are five times more likely to suffer a stroke than someone with a normal heart […]
Sotera Wireless to Showcase Newest ViSi Mobile® 1.5G Capability at AONE Conference in April
SAN DIEGO–(BUSINESS WIRE)–Sotera Wireless, maker of the ViSi Mobile® Surveillance Monitoring System and a pioneer in continuous vital signs monitoring, announced it will be exhibiting at the American Organization of Nurse Executives (AONE), the leading voice of nursing leadership in health care. The 4-day event brings nurse leaders together to […]