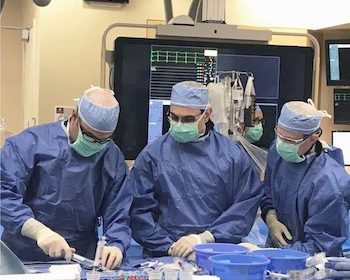
JACKSON, Mich., June 26, 2019 /PRNewswire/ — Doctors performed the first catheter-based replacement of an aortic heart valve today at Henry Ford Allegiance Health, part of the nationally recognized transcatheter aortic valve replacement (TAVR) program of Henry Ford Health System. TAVR is a minimally invasive alternative to open-heart valve replacement surgery. The first Henry […]
Other News
VIVO™ System Cleared by FDA
MT. OLIVE, N.J., June 26, 2019 /PRNewswire/ — Catheter Precision, Inc., announced today that the U.S. Food and Drug Administration (FDA) has cleared its new VIVO™ (View into Ventricular Onset) system for market release in the United States. VIVO is a pre-procedure planning tool that offers 3D cardiac mapping to aid in localizing […]
Medtronic Announces the Upsizing of its Maximum Tender Offer to up to $4.35 billion for Certain Outstanding Debt Securities Issued by Medtronic, Inc., Medtronic Global Holdings S.C.A. and Covidien International Finance S.A.
DUBLIN, June 25, 2019 (GLOBE NEWSWIRE) — Medtronic plc (the “Company”) (NYSE:MDT) today announced the upsizing of the previously announced cash tender offer (the “Maximum Tender Offer”) by its wholly-owned indirect subsidiaries, Medtronic, Inc., Medtronic Global Holdings S.C.A. (“MGH”) and Covidien International Finance S.A. (“CIFSA” and, together with Medtronic, Inc. […]
Boston Scientific Presents Strategies for Sustained Long-Range Growth Targets at 2019 Investor Day
MARLBOROUGH, Mass., June 26, 2019 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) is hosting a meeting with the investment community today in New York City to review its business and long-term growth strategies. The company is presenting plans for its product pipeline and strategic investments to enable improved clinical and economic outcomes, sustain category leadership […]
Neovasc Regains Compliance with Nasdaq Minimum Market Value Rule
VANCOUVER, June 25, 2019 /PRNewswire/ – Neovasc Inc. (“Neovasc” or the “Company”) (NASDAQ:NVCN) (TSX: NVCN), a leader in the development of minimally invasive transcatheter mitral valve replacement technologies and in the development of minimally invasive devices for the treatment of refractory angina, announced today that it has received written notification (the “Nasdaq […]
East Bay Cardiovascular and Thoracic Associates Partners with Health Prime to Get Revenue Cycle Management Back on Track
NATIONAL HARBOR, Md.–(BUSINESS WIRE)–Health Prime, a leading provider of revenue cycle management services, today announced that East Bay Cardiovascular and Thoracic Associates, a premier multi-specialty surgical group, has signed a partnership agreement for revenue cycle management services. Health Prime was brought onboard to scale operations and optimize current revenue opportunities. […]
Catasys Appoints Healthcare Executive Daniel Prewitt as Senior Vice President of Sales
LOS ANGELES–(BUSINESS WIRE)–Catasys, Inc. (NASDAQ: CATS) (“Catasys” or the “Company”), a leading AI and technology-enabled healthcare company, today announced the appointment of Daniel Prewitt as the senior vice president of sales. In this role, Prewitt will be responsible for leading the Company’s sales to health plans and self-funded employers. Bringing over […]
BioSyent Announces Withdrawal of Health Canada Submissions for Cardiovascular Products
TORONTO, June 26, 2019 (GLOBE NEWSWIRE) — BioSyent Inc. (“BioSyent”, TSX Venture: RX) announces that it has informed Health Canada that it will be withdrawing its New Drug Submissions for two cardiovascular products, subsequent to the Notice of Deficiency received from Health Canada earlier this year in respect of these […]
Amgen And MBC BioLabs Announce Winners Of The Amgen Golden Ticket
SAN FRANCISCO, June 25, 2019 /PRNewswire/ — Amgen (NASDAQ:AMGN) and MBC BioLabs (formerly QB3@953) today announced that Regencor and Nitrome Biosciences have each been awarded an Amgen Golden Ticket to MBC BioLabs. The companies receive priority admission or renewal for one year of lab bench space and access to core facilities at the MBC BioLabs […]
Samuel E. Navarro Appointed to BioSig Board of Directors
Santa Monica, CA, June 25, 2019 (GLOBE NEWSWIRE) — BioSig Technologies, Inc. (NASDAQ: BSGM), a medical device company developing a proprietary biomedical signal processing technology platform designed to address an unmet need for the electrophysiology (EP) marketplace, today appointed Samuel E. Navarro to its Board of Directors. Mr. Navarro brings to BioSig […]