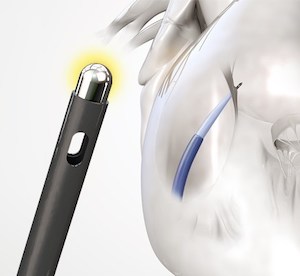
TORONTO, June 19, 2019 /PRNewswire/ – A cost-effectiveness analysis completed by the University of California, San Francisco (UCSF) and Baylis Medical has shown that use of Baylis’ RF transseptal needle results in cost savings per procedure, compared to use of a mechanical transseptal needle. The study, entitled A Cost-Effectiveness Analysis Comparing the Baylis RF Needle to […]
Other News
Bardy Diagnostics™ Selected as One of 50 Leading Startups in the 2019 MedTech Innovator Showcase Program
SEATTLE, June 19, 2019 /PRNewswire/ — Bardy Diagnostics, Inc., (“BardyDx”), a leading provider of ambulatory cardiac monitoring technologies and custom data solutions, announced that MedTech Innovator, the premier nonprofit startup accelerator in the medical technology industry, has selected BardyDx to participate in the organization’s 2019 Showcase program, featuring the most promising medical technologies from […]
New Studies Suggest That DiA’s AI-based Software, LVivo EF, Is Accurate and Efficient in Assessing Ejection Fraction Values Compared to Cardiac MRI and Contrast Echocardiography
BEER-SHEVA, Israel, June 19, 2019 /PRNewswire/ — DiA Imaging Analysis, the developers of artificial intelligence (AI) powered ultrasound analysis solutions, has announced today the presentation of two studies assessing the performance and accuracy of the company’s LVivo EF AI-based solution that generates fully automated Left Ventricular (LV) Ejection Fraction (EF). EF is a […]
Philips receives FDA premarket approval for its HeartStart OnSite and HeartStart Home defibrillators
Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced that the Center for Devices and Radiological Health (CDRH) of the Food and Drug Administration (FDA) has approved the company’s premarket approval (PMA) application for its HeartStart OnSite defibrillator [1] and HeartStart Home defibrillator [2], […]
PAI Health Joins American Heart Association Innovators Network to Scale Digital Solutions that Improve Cardiovascular Health
VANCOUVER, British Columbia, June 18, 2019 (GLOBE NEWSWIRE) — PAI Health, a heart health software company, will join the American Heart Association’s Center for Health Technology & Innovation (CHTI) Innovators Network to contribute their science-based technology that helps reduce the risk of cardiovascular disease, the leading cause of death for all […]
Stereotaxis Announces the First Graduating Class of the Robotic Electrophysiology Fellows Program
ST. LOUIS, June 18, 2019 (GLOBE NEWSWIRE) — Stereotaxis, Inc. (OTCQX: STXS), the global leader in innovative robotic technologies for the treatment of cardiac arrhythmias, today announced the first graduating class from its Robotic Electrophysiology Fellows Program. The Robotic Electrophysiology Fellows Program is designed to enhance the traditional electrophysiology fellowship […]
CardioFocus Launches 100th HeartLight® Center Worldwide
MARLBOROUGH, Mass., June 18, 2019 /PRNewswire/ — CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for atrial fibrillation (AFib), announced today that the HeartLight® Endoscopic Ablation System is now offered at more than 100 hospitals worldwide. The HeartLight System is a revolutionary catheter ablation technology for controlled and consistent pulmonary vein isolation (PVI), the […]
Zoll Medical Corporation Acquires TherOx, Inc.
CHELMSFORD, Mass.–(BUSINESS WIRE)–ZOLL® Medical Corporation, an Asahi Kasei Group Company that manufactures medical devices and related software solutions, today announced it has acquired TherOx, Inc., of Irvine, Calif., a privately held company. TherOx is focused on improving treatment of acute myocardial infarction (AMI) and markets systems to deliver SuperSaturated Oxygen (SSO2) […]
Canon Medical Receives FDA Clearance for Deep Convolutional Neural Network Image Reconstruction Technology for CT
TUSTIN, Calif.–(BUSINESS WIRE)–Building on its advanced image reconstruction technologies, Canon Medical Systems USA, Inc. has received 510(k) clearance on its new deep convolutional neural network (DCNN) image reconstruction technology, ushering in a new era for CT. Canon Medical’s Advanced Intelligent Clear-IQ Engine (AiCE) uses a deep learning algorithm to differentiate signal from […]
Journal of Endovascular Therapy Publishes Peer Reviewed Article Underscoring the Clinical Impact of Post-Angioplasty Dissections
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today welcomed the peer-reviewed publication, “Dissections After Infrainguinal Percutaneous Transluminal Angioplasty (PTA): Systematic Review and Current State of Clinical Evidence” currently available online, with the print article scheduled to be published in the August issue […]