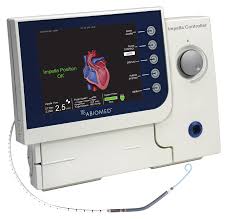
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ:ABMD) announces the U.S. FDA has approved the expansion of the Impella 5.0 and Impella LD PMA labeling for the treatment of cardiogenic shock. The expansion extends the duration of support for each pump from 6 days to 14 days. The Impella 5.0 and the Impella LD are forward flow heart pumps […]
Other News
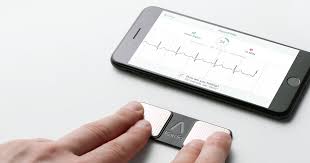
FDA Grants First Ever Clearance For Six-Lead Personal ECG Device
MOUNTAIN VIEW, Calif., May 13, 2019 /PRNewswire/ — AliveCor, the leader in FDA-cleared consumer electrocardiogram technology (ECG), today announced its third FDA clearance in three months, making KardiaMobile 6L the world’s first available six-lead personal ECG device. This highly anticipated clearance gives patients and their physicians an even more detailed view into patients’ […]
MyoKardia Reports First Quarter 2019 Financial Resul
Announces Enrollment Completing in the Phase 2 MAVERICK-HCM Trial Company to Host Conference Call and Webcast Today at 4:30 p.m. ET (1:30 p.m. PT) SOUTH SAN FRANCISCO, Calif., May 09, 2019 (GLOBE NEWSWIRE) — MyoKardia, Inc. (Nasdaq: MYOK), a clinical-stage biopharmaceutical company pioneering a precision medicine approach for the treatment […]
NEW STUDIES HIGHLIGHT UNDERUSE OF IMPLANTABLE CARDIAC DEVICES
DUBLIN and SAN FRANCISCO – May 10, 2019 – Three new studies show that patients who are medically indicated for implantable heart devices, including implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy (CRT), often do not receive needed therapy. The findings underscore gender and race treatment disparities, as well as a […]
Quantum Genomics Announces the Publication of a New Scientific Article in the Journal Hypertension
PARIS and NEW YORK, May 10, 2019 (GLOBE NEWSWIRE) — Quantum Genomics (Euronext Growth – FR0011648971 – ALQGC), a biopharmaceutical company specializing in the development of a new drug class that directly targets the brain to treat resistant hypertension and heart failure, announces the publication of a new scientific article in the […]
CardioFocus Announces Impressive Results From Pivotal Confirmatory Study With The Breakthrough HeartLight® X3 System
MARLBOROUGH, Mass., May 10, 2019 /PRNewswire/ — CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for atrial fibrillation (AFib), today announced the presentation of results from its pivotal confirmatory study evaluating the HeartLight X3 System for the treatment of AFib. The results, which demonstrated superior procedural times and impressive procedural […]
BD Announces Results for 2019 Second Fiscal Quarter; Updates Fiscal 2019 Guidance
– As reported, revenues of $4.195 billion decreased 0.6 percent. – On a comparable, currency-neutral basis, revenues increased 3.4 percent. – As reported, diluted earnings per share of $(0.07) increased 63.2 percent. – As adjusted, diluted earnings per share of $2.59 decreased 2.3 percent, and increased 7.2 percent on a […]
Neovasc Announces First Quarter 2019 Financial Results
NASDAQ, TSX: NVCN Recent Highlights Announced the 1,000th recipient of a Neovasc ReducerTM (“Reducer”) implant Company sponsored Reducer Symposium attended by over 100 attendees at the 85th Annual Conference of the German Society of Cardiology (“DGK”) Completed the conceptual work for the TF/TS TiaraTM (“Tiara”) system; planning to initiate a small clinical feasibility […]
Embolx Extends the Sniper Balloon Occlusion Microcatheter Family with Launch of New K-Tip Design
SUNNYVALE, Calif.–(BUSINESS WIRE)–Embolx, Inc., a medical device company developing advanced microcatheters for arterial embolization procedures, today announced the commercial availability of the new Sniper® K™-tip in the United States and Europe. The Sniper Balloon Occlusion family of microcatheters now includes the new K-tip, a 45-degree angled tip designed to enhance […]
Spacelabs Debuts Sentinel 11 With Enhanced Patient Data Security and Accessibility for Cardiologists
SNOQUALMIE, Wash.–(BUSINESS WIRE)–Spacelabs Healthcare® (the “Company” or “Spacelabs”) debuted Sentinel® 11 at the 40th Annual Heart Rhythm Scientific Sessions in San Francisco. This latest release from Spacelabs advances cardiology information management with optimized workflows, expanded connectivity, and robust data security capabilities. “As healthcare organizations face increasingly sophisticated cybersecurity threats, the […]