PALO ALTO, Calif., Oct. 30, 2017 /PRNewswire-USNewswire/ — ReCor Medical announced progress milestones in the study of its Paradise® Renal Denervation System as a potential treatment for hypertension, including the 1000th consented and the 150th randomized subjects in the RADIANCE-HTN study, and publication of a study design review in the American Heart […]
Coronary/Structural Heart
Abbott Introduces Next Generation of Most Widely Used Heart Stent for People With Coronary Artery Disease in Europe
ABBOTT PARK, Ill., Oct. 30, 2017 /PRNewswire/ — Abbott today announced it received CE Mark for XIENCE Sierra, the newest generation of the company’s gold-standard XIENCE everolimus-eluting coronary stent system. CE Mark allows sale of the device in the European Union and other countries that recognize CE Mark. Advances in this generation […]
Early-Stage Medtech HighLife Nabs $14M for Transcatheter Mitral Valve Replacement
PARIS–(BUSINESS WIRE)– HighLife, an early-stage medtech company focused on the development of a unique trans-catheter mitral valve replacement (TMVR) system to treat patients suffering from mitral regurgitation, announced today the closing of a €12.3 million investment round led by Sofinnova Partners which becomes the main investor in the company. HighLife had previously secured financing from […]
CathWorks Ltd. Appoints James M. Corbett Chief Executive Officer
KFAR SABA, Israel, Oct. 27, 2017 /PRNewswire/ — CathWorks Ltd., a leader in the development of non-invasive Fractional Flow Reserve (FFR) measurements for guiding coronary interventions, announced today the appointment of James M. Corbett as its Chief Executive Officer. Corbett, an industry veteran with broad experience in global commercialization, has served as President, Boston Scientific International, […]
Nitiloop Announces FDA 510(k) Clearance for its Nova Cross Extreme and Nova Cross BTK Micro catheter for Support of Guidewire Access to Discrete Regions of the Coronary and Peripheral Vasculature
NETANYA, Israel, October 26, 2017 /PRNewswire/ — Nitiloop, a medical device company dedicated to the development of Cardiovascular and peripheral microcatheters for complex lesions, received FDA clearance for its new Nova Cross™ Extreme and Nova Cross™ BTK. These dedicated microcatheters are joining the Nova Cross™ product family combining innovative low profile microcatheter technology with uniquely designed Nitinol scaffold providing […]
Medinol Ltd. Receives CE Mark for the EluNIR™ Ridaforolimus-Eluting Coronary Stent System, and announces first commercial implantations
Medinol, a global Interventional Cardiology device company, announced that it obtained CE-mark for EluNIR™, its novel Drug Eluting Stent featuring a unique stent design with low metal footprint and a proprietary elastomeric coating, both designed to optimize clinical outcomes. EluNIR demonstrated outstanding outcomes in two randomized pivotal studies. In BIONICS, a more-comers […]
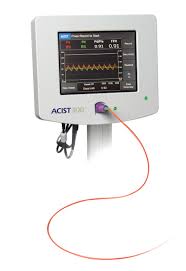
ACIST Medical Systems Announces Approval and Launch of Next-Generation FFR System
DENVER & EDEN PRAIRIE, Minn.–(BUSINESS WIRE)–ACIST Medical Systems, Inc., a Bracco Group Company, today announced the global launch of its ACIST RXi® Mini™ System, the next generation system of its RXi Rapid Exchange FFR System. The RXi Mini System will debut at the 29th Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium […]
Novoheart Strengthens North American Presence Opening New R&D Location at the World-class Cove Facility, UC Irvine, California
IRVINE, CALIFORNIA — (Marketwired) — Oct 25, 2017 — Novoheart (“Novoheart” or the “Company”) (TSX VENTURE: NVH), a global stem cell biotechnology company, is pleased to announce that it has opened a new US location in The Cove at UC Irvine, California. The Cove is home to UCI Applied Innovation – a public-private […]
ROX Medical Announces Sustained Improvements in Patients With Uncontrolled Hypertension Treated With the ROX Coupler
SAN CLEMENTE, Calif., Oct. 26, 2017 /PRNewswire/ — Rox Medical Inc., a privately held medical device company pioneering an innovative interventional vascular therapy for uncontrolled hypertension, announced the publication of 12-month outcomes of the ROX CONTROL HTN study in the prestigious journal, Hypertension. CONTROL HTN is a multi-center randomized controlled trial that […]
CorInnova Awarded Seminal Patent for Minimally Invasively-Delivered Soft Robotic Heart Device to Support Heart Function
HOUSTON–(BUSINESS WIRE)–October 26, 2017– CorInnova Inc., an emerging medical device company developing novel technology for the treatment of Heart Failure, announced today three milestone events: (1) the Company has received notice of allowance of a seminal patent to protect its intellectual property associated with the world’s first minimally invasively-delivered soft robotic […]